Ubiquitin and lts Derivatives
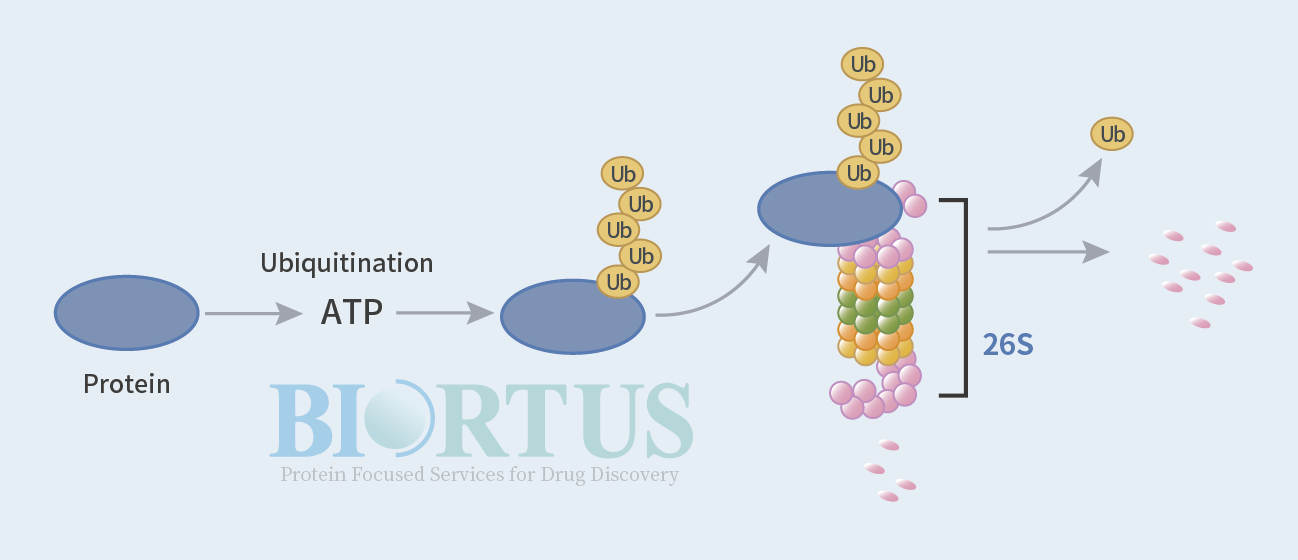
Ubiquitin is a small protein present in most eukaryotic cells. Its main function is to tag proteins that need to be broken down for hydrolysis. When a protein tagged with ubiquitin moves to the barrel-shaped protease, the protease will hydrolyze the protein. Ubiquitin can also tag transmembrane proteins, such as receptors, and remove them from the cell membrane.
Ubiquitin consists of 76 amino acids and has a molecular weight of approximately 8,500 daltons. It is highly conserved in eukaryotes, with human and yeast ubiquitin showing 96% similarity. The human genome contains approximately 19,000 coding genes. After post-translational splicing and modification, hundreds of thousands of proteins can be produced, including cellular structural proteins, hormones, enzymes, transcription factors, etc., which orderly regulate life activities. Protease degradation, for example, trypsin digests large food proteins in the small intestine into small peptides and amino acids, which are then absorbed by the small intestine. Endocytosis engulfs foreign proteins into the cell, where they are digested by the lysosomal digestive enzymes in the food vacuole without consuming energy.
 Drug Targets
Drug Targets

