Biortus one-stop service supports the research and development of new drugs for China Innovation Center of Roche (CICoR), published in Nature Communications
Release Time:
2024-06-14 11:28
Source:
On April 11, 2024, Nature Communications published online an article of China Innovation Center of Roche (CICoR) in collaboration with Biortus, introducing the cryo-EM structure of the dimeric human parainfluenza virus type 3 (hPIV3) L-P complex. The structural basis of non segmented negative stranded RNA virus (nsNSVs) polymerase L-L dimerization has been revealed for the first time, and functional experiments have shown that polymerase dimerization is crucial for virus genome replication.
HPIV3, one of four hPIV subtypes, is a common cause of severe respiratory infections in infants and children. The transcription and replication of nsNSVs are completed by the same multifunctional polymerase complex, which is composed of the large proteins (L) and cofactors such as phosphoproteins (P), thus serving as an important target for the development of antiviral drugs.
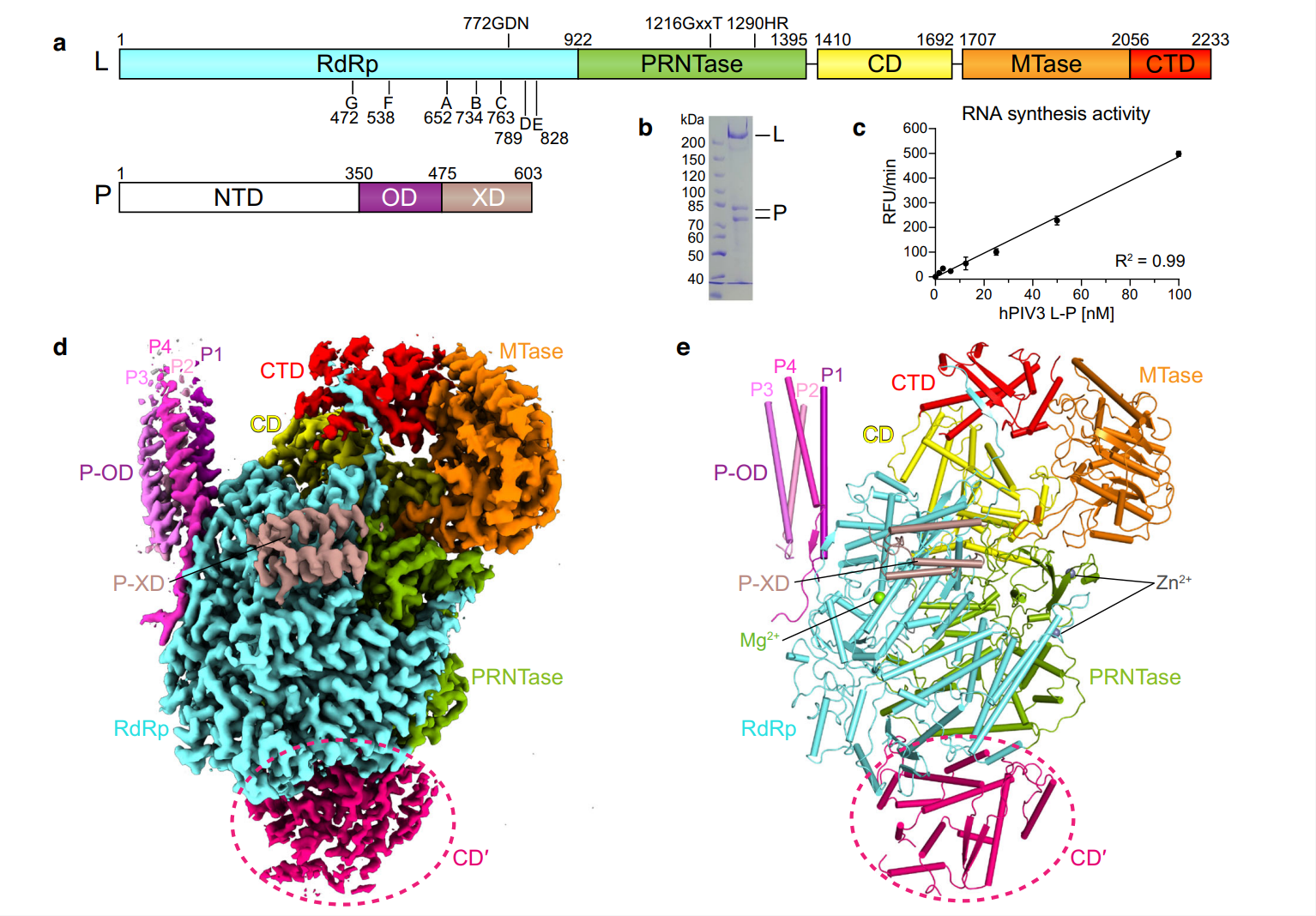
This research provides a significant breakthrough in the field of molecular mechanisms of nsNSV RNA replication, and also suggests a potential new avenue for antiviral drug design. Biortus provides one-stop structural biology research services for China Innovation Center of Roche, including hPIV3 L-P plasmid design, protein expression and purification, biophysical analysis, and cryo-EM structure determination.


